Proteins unfold by breaking electrical contacts
02. Dezember 2015
über
über
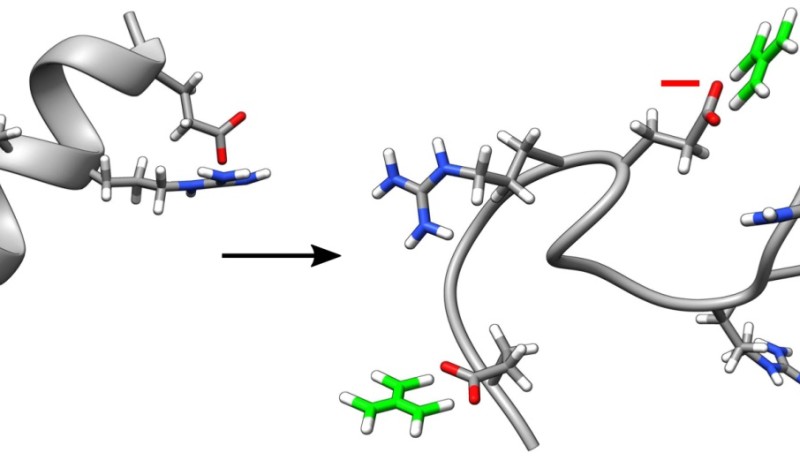
Researchers from the University of Amsterdam have discovered that the substance guanidinium chloride unfolds proteins by breaking the electrical contacts that keep a protein molecule together. The discovery is important for a better understanding of the forces that keep a protein in the folded, i.e. healthy state. The article about the discovery will be published online on 2 December and on 8 December in the Journal Angewandte Chemie International Edition.
Charged atoms in the chain
Protein molecules are long chains of amino acids. Such a chain is usually neatly 'folded' in a well-defined, compact structure. Proteins are only biologically active in this compact form. Sometimes, certain proteins unfold spontaneously. This plays an important role in many diseases. To understand how the unfolding takes place, researchers in a biochemistry lab often added guanidinium chloride (named after guano, bird droppings, from which the substance was first prepared in the 19th century) to the water in which the proteins are dissolved. The guanidinium ions (charged molecules) in this substance are very efficient at unfolding protein molecules. How guanidinium ions do this is still largely unknown. ERC PhD student Heleen Meuzelaar and FOM PhD student Matthijs Panman (now postdoc in Gothenburg) discovered that the guanidinium ions displace other positively charged atoms.
Salt bridge
The compact structure of proteins is kept together by various forces. One of these is the electrical force: most proteins contain positively and negatively charged atoms in the amino acid chains, arranged in such a way that in the folded state, pairs of positive and negative charges are located opposite each other. The attractive electrical force between the opposite charges in the pair neatly keeps the protein in the folded structure. Such an electrical contact between two opposite charges in the protein is called a salt bridge (because this is also how salt crystals are held together: think about the opposite charges of sodium and chloride ions in table salt).
Meuzelaar and Panman investigated the effect of guanidinium on the salt bridges. In a series of biochemical experiments, they first examined the effect of guanidinium on a series of different mini-proteins (peptides). Each time, they compared mini-proteins that were identical in terms of composition and size, but where the position of the oppositely charged atoms in the chain was different. A small change in the position of the charged atoms in the chain was found to have a huge effect on how well the guanidinium unfolded the mini-proteins. In some mini-proteins, guanidinium even facilitated the folding of the protein instead of its unfolding.
Ions replace positive atoms
Using infrared laser techniques, the researchers could see what guanidinium does with the salt bridges. It seems that the guanidinium ions, which are positively charged, replace the positive atoms in the salt bridges: they displace the positive atoms, as a result of which the salt bridges break and the protein unfolds. A new salt bridge is then formed: no longer between the two oppositely charged atoms of the protein, but between the positively charged guanidinium and the negatively charged atom of the protein.
The discovery is important for a better understanding of the forces that protect proteins from spontaneous unfolding. It is suspected that such spontaneous unfolding is the first step in the development of geriatric diseases such as Alzheimer's and Parkinson's.
Contact
Sander Woutersen, , +31 20 525 70 91.
Homepage
Reference
The full article can be found here.
Charged atoms in the chain
Protein molecules are long chains of amino acids. Such a chain is usually neatly 'folded' in a well-defined, compact structure. Proteins are only biologically active in this compact form. Sometimes, certain proteins unfold spontaneously. This plays an important role in many diseases. To understand how the unfolding takes place, researchers in a biochemistry lab often added guanidinium chloride (named after guano, bird droppings, from which the substance was first prepared in the 19th century) to the water in which the proteins are dissolved. The guanidinium ions (charged molecules) in this substance are very efficient at unfolding protein molecules. How guanidinium ions do this is still largely unknown. ERC PhD student Heleen Meuzelaar and FOM PhD student Matthijs Panman (now postdoc in Gothenburg) discovered that the guanidinium ions displace other positively charged atoms.
Salt bridge
The compact structure of proteins is kept together by various forces. One of these is the electrical force: most proteins contain positively and negatively charged atoms in the amino acid chains, arranged in such a way that in the folded state, pairs of positive and negative charges are located opposite each other. The attractive electrical force between the opposite charges in the pair neatly keeps the protein in the folded structure. Such an electrical contact between two opposite charges in the protein is called a salt bridge (because this is also how salt crystals are held together: think about the opposite charges of sodium and chloride ions in table salt).
Meuzelaar and Panman investigated the effect of guanidinium on the salt bridges. In a series of biochemical experiments, they first examined the effect of guanidinium on a series of different mini-proteins (peptides). Each time, they compared mini-proteins that were identical in terms of composition and size, but where the position of the oppositely charged atoms in the chain was different. A small change in the position of the charged atoms in the chain was found to have a huge effect on how well the guanidinium unfolded the mini-proteins. In some mini-proteins, guanidinium even facilitated the folding of the protein instead of its unfolding.
Ions replace positive atoms
Using infrared laser techniques, the researchers could see what guanidinium does with the salt bridges. It seems that the guanidinium ions, which are positively charged, replace the positive atoms in the salt bridges: they displace the positive atoms, as a result of which the salt bridges break and the protein unfolds. A new salt bridge is then formed: no longer between the two oppositely charged atoms of the protein, but between the positively charged guanidinium and the negatively charged atom of the protein.
The discovery is important for a better understanding of the forces that protect proteins from spontaneous unfolding. It is suspected that such spontaneous unfolding is the first step in the development of geriatric diseases such as Alzheimer's and Parkinson's.
Contact
Sander Woutersen, , +31 20 525 70 91.
Homepage
Reference
The full article can be found here.
Mehr anzeigen
Weniger anzeigen


Diskussion (0 Kommentare)