A Designer's Guide to Lithium Battery Charging
25. September 2012
über
über
By Steven Keeping
Contributed By Electronic Products
2012-09-25
However, it has been a tough design challenge to get the technology to where it is today. Lithium is a highly reactive material that can, for example, burst into flames if it comes into contact with water. Engineers and scientists have worked hard to develop novel compounds that can leverage the advantages of lithium while producing inexpensive, reliable, and safe batteries.
Nonetheless, overcharging a modern Li-ion cell can cause permanent damage, or worse, instability and potential danger. To avoid this problem, Li-ion batteries need to be charged following a carefully controlled constant current/constant voltage regime that is unique to this cell type.
This article describes the structure of modern Li-ion batteries, explains how they work, and why they need to be charged using this special profile. The article will then show how chip manufacturers have designed integrated power management chips that make it easier for an engineer to design a Li-ion battery charger for his or her next product.
Background
Rechargeable Li-ion batteries are the mainstay of the portable device industry. Without them, portable devices would not be so portable, due to the additional bulk and weight of alternative batteries. Developing the technology, such that manufacturers can produce hundreds of millions of reliable Li-ion batteries every year, has been a long, tough haul.
Early attempts in the 1970s used titanium sulphide as the positive electrode and pure lithium metal as the negative. Titanium sulphide is an intercalation compound. Such compounds are materials with a layered crystalline structure that allow atoms, ions, or molecules of other materials to migrate and then reside between the layers. During discharge, lithium ions moved from the negative electrode to the positive. Charging forced them to move back the other way. Unfortunately, after many cycles the lithium electrode formed dendrites, rough “spikes” of the pure metal that were highly reactive and could cause fire or even explosions.
The breakthrough came with the development of alternative lithium-based intercalation compound to provide a source of lithium ions to replace the pure lithium metal electrode. After many experiments, scientists settled on Lithium-cobalt-oxide (LiCoO2) — a material that is stable in air.
The basics
All types of rechargeable batteries, whether lead-acid, nickel-metal hydride (NiMH), nickel-cadmium (NiCd), lithium-ion (Li-ion), or other more exotic types such as nickel-hydrogen (NiH2) or lithium-iron phosphate (LiFePO4) operate according to the same principle.
Each type exploits a reversible electrochemical reaction. Charging is achieved by applying a current that stores energy in the cell to be released later by the reverse reaction.
Lithium-based technology is particularly appealing for two key reasons: Lithium is the most electropositive metal (i.e. it exhibits a high positive charge), lending itself to batteries with higher voltages than other rechargeable types (around 3.6 V compared to 1.2 to 1.5 V for nickel-based batteries); and it is the lightest metal (in fact, only two elements, hydrogen and helium, are lighter) allowing it to store more energy per kilogram than other metals (around 3,900 Ahr/kg compared to 260 Ahr/kg for lead).1
Lithium ions are used in modern batteries because they are less reactive than the element’s atoms, making the battery much safer.
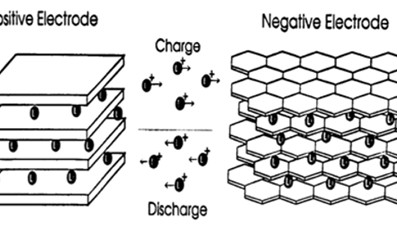
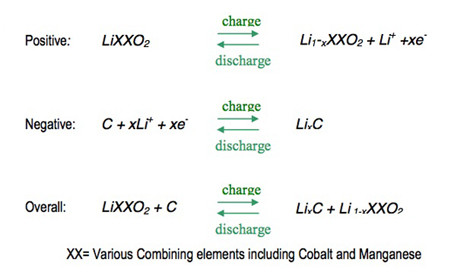
Today’s Li-ion batteries use two intercalation compounds for the positive and negative electrodes. LiCoO2 forms the positive electrode, while graphite is used for the negative one.
Applying a current to the battery causes the ions to move from the cobalt-oxide lattice into the graphite one (Figure 1).2 In the process, positive material is oxidized and the negative material is reduced. The move from one electrode to another increases the ion’s potential energy. When the battery is used to power a device, the ions migrate the other way, releasing the energy stored during the original reaction. Figure 2 illustrates the process.3
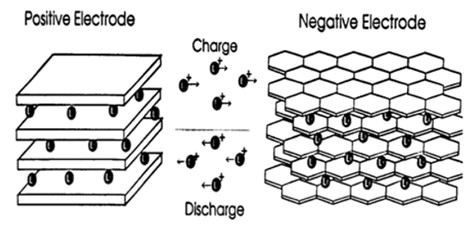
It sounds like a straightforward process, but there are dangers; the major ones occur if the battery is overcharged. The inventors of the contemporary Li-ion battery, Sony, discovered that overcharging could remove so many lithium ions from the cobalt-oxide lattice that it disintegrates. And if too many ions are packed into the graphite, there is a danger that small amounts of lithium metal can form which can make the battery unstable and potentially dangerous.
The flexible battery
In early versions of Li-ion batteries, the electrodes were separated by a liquid electrolyte. But one novel improvement for later Li-ion batteries was the use of new separators that incorporated the electrolyte through which the ions moved.
Later versions used a porous separator soaked in an electrolytic gel. This allowed the batteries to be made using a sandwich construction leading to the thin designs common to today’s svelte mobile handsets (Figure 3). Further development led to lithium-polymer (Li-polymer) cells that used a solid polymer instead of a liquid electrolyte or gel as the separator.

Li-polymer batteries have a number of advantages over conventional Li-ion cells. They are cheaper to manufacture, can be made into virtually any shape (allowing manufacturers to make products with exotic form factors), and tend to be more rugged. One downside is that the ions travel more slowly through the solid polymer than liquid electrolyte, so charging takes longer unless the polymer is very thin.
Li-ion charging regime
The fragile tendency of Li-Ion batteries to overcharging imposes stringent recharge requirements. But it is not just overcharging that is the problem, because even slight undercharging significantly reduces capacity. Undercharging the battery by 1.2 percent of its optimum full-charge voltage, for example, lowers the capacity by a remarkable 9 percent. Consequently, designers aim to charge the cell to within 1 percent of its optimum full-charge to get the most out of the battery.4
Modern charger circuits typically apply a regulated constant current into the battery and end by forcing the gradually decreasing current necessary to charge the battery to a regulated full-charge voltage. A schematic of this type of charger is shown in Figure 4. Such a charging regime is known as a constant-current/constant-voltage (CC/CV) regime.
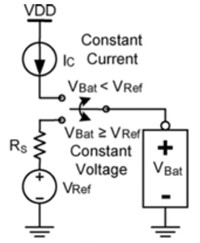
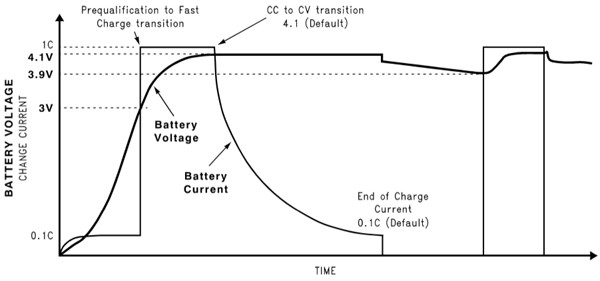
If the battery is deeply discharged (for example, to below 3 V) a small “pre-conditioning” charge – around 10 percent of the full charge current – is applied to stop the cell overheating until it is able to accept the full current of the constant current phase. During the constant current phase, the battery is charged at 1 C or less, (where 1 C equals the maximum current the battery can supply for one hour; for example, for a 2 Ah battery, C = 2 A). The constant current phase continues until the battery voltage reaches 4.1 V.
(It should be noted that rapid charging to 4.1 V results in a lower battery capacity at the end of the first stage than slower charging. For example, charging at 0.7 C, although accelerating the initial phase, results in a capacity of 50 to 70 percent when 4.1 V is reached, whereas charging at less than 0.18 C takes much longer but can result in a full battery as soon as the voltage reaches 4.1 V.)
When the battery voltage reaches 4.1 V, the charger switches to a constant voltage phase to avoid the risk of overcharging. Superior battery chargers manage the transition from constant current to constant voltage smoothly to ensure maximum capacity is reached without risking damage to the battery.
During the constant voltage phase, the current drops until it reaches around 0.1 C when charging is terminated. If the charger is left connected to the battery, a periodic “top up” charge is applied to counteract battery self discharge. The top up charge is typically initiated when the open circuit voltage of the battery drops to less than 3.9 to 4 V and terminates when the full charge voltage of 4.1 to 4.2 V is again attained.5
The phases of a typical Li-ion charging regime are illustrated in Figure 5.
Battery-charging solutions
Many semiconductor vendors manufacture chips that manage the Li-ion battery-charging process. Using these devices simplifies the design of either standalone battery chargers, or integrated charging circuitry as part of the end product.
National Semiconductor, for example, offers its LM3658 single-chip charger IC for handheld applications. The chip can safely charge and maintain a single-cell Li-ion/Li-polymer battery operating from an AC wall adapter or USB power source.
The LM3658 operates in five modes: pre-qualification mode, constant-current mode, constant-voltage mode, top-off mode, and maintenance mode. The company claims that optimal battery management is obtained through thermal regulation, battery temperature measurement, and multiple safety timers.
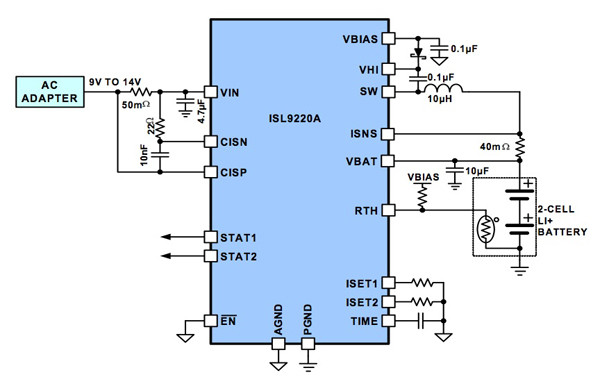
For its part, Intersil supplies the ISL9220. The company says this is a cost-effective and versatile battery charger for one- and two-cell Li-ion- and Li-polymer-based portable applications. The device features synchronous pulse width modulation (PWM) technology, which is said to maximize power efficiency, minimizing charge time and heat. External resistors program the fast charge and end-of-charge currents. Additional features include preconditioning of an over-discharged battery, automatic recharge, and thermally enhanced QFN package. Figure 6 shows a typical application circuit for a two-cell Li-ion battery.
Maxim’s MAX1551 device charges a single-cell Li-ion battery using no external FETs or diodes, and accepts operating input voltages up to 7 V. The company says on-chip thermal limiting simplifies board layout and allows an optimum-charging rate without the thermal limits imposed by worst-case battery and input voltage. When DC power is connected, charging current is set at 280 mA typ. No input-blocking diodes are required to prevent battery drain.
Several other companies manufacture fully-integrated Li-ion battery chargers, including Fairchild Semiconductor, Linear Technology,STMicroelectronics, and Texas Instruments.
A work in progress
The development of Li-ion batteries – culminating in the thin, light, and reliable power sources for modern handheld devices and other mobile devices seen today – has been a decades-long task for dedicated engineers and scientists. Nonetheless, the technology is still far from mature, and current developments are focused on improved intercalation materials to maximize the energy density promised by lithium’s high electro-potential.
To get the most from contemporary Li-ion power packs, and to avoid overcharging which damages the cell and can be dangerous, charging must be done carefully and follow an established CC/CV profile. However, engineers choosing Li-ion power for their mobile designs can simplify “smart” charger design by basing their charger on one of the many integrated battery management chips from leading silicon vendors.
References:
- “Hooked on Lithium,” The Economist, June 2002.
- “Comments on the History of Lithium-Ion Batteries,” Ralph J. Brodd, Broddarp of Nevada, Inc.
- Harding Battery Handbook, Harding Energy Inc., January 2004.
- “Accurate, Compact, and Power-Efficient Li-Ion Battery Charger Circuit,” IEEE Transactions on Circuits and Systems - II: Express Briefs, Vol. 53, No. 11. Min Chen and Gabriel A. Rinco?n-Mora, November 2006.“Challenges in Li-ion charging,” David Hamo, Semtech, Electronic Products, August 2003.
Disclaimer: The opinions, beliefs, and viewpoints expressed by the various authors and/or forum participants on this website do not necessarily reflect the opinions, beliefs, and viewpoints of Digi-Key Corporation or official policies of Digi-Key Corporation.
Mehr anzeigen
Weniger anzeigen


Diskussion (0 Kommentare)